Table of Contents
A Phase 1/2, Open-label Assessment Of The Safety ... - Scanr now in NYC - limited period only
Publisher Copyright: extcopyright 2021, The Author(s), under exclusive licence to Springer Nature Switzerland AG. Copyright: Copyright 2021 Elsevier B.V., All rights reserved.", year = "2021", month = oct, doi = "10. 1007/s40266-021-00882-2", language = "English", volume = "38", pages = "887--910", journal = "Drugs and Aging", issn = "1170-229X", publisher = "Adis International Ltd", number = "10",TY - JOURT1 - Safety and Tolerability of Natural and Synthetic Cannabinoids in Older Adults, T2 - A Systematic Review and Meta-Analysis of Open-Label Trials and Observational Studies, AU - Pisani, Sara, AU - Mc, Goohan, Katie, AU - Velayudhan, Latha, AU - Bhattacharyya, Sagnik, N1 - Funding Information: SB and LV received funding from Parkinson’s UK (grant number G-1901).
16/126/53]. The authors acknowledge support from the National Institute for Health Research Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. No other disclosures were reported.
Copyright: Copyright 2021 Elsevier B.V., All rights reserved. PY - 2021/10Y1 - 2021/10N2 - BACKGROUND AND OBJECTIVE: Although cannabinoid-based medications are increasingly used by older adults, their safety and tolerability in this age group remain unclear. The purpose of this systematic review was to examine the safety and tolerability of cannabinoid-based medications by conducting a meta-analysis of open-label observational studies of cannabinoid-based medications for all indications in individuals with a mean age of ≥50 years.
Buy Cannabis In Parkinson's Disease — The Patient's Perspective ... in TEXAS
Study quality was assessed using an adapted version of the Grading of Recommendations Assessment, Development and Evaluation criteria and Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed. We included studies that (a) were published from 1990 onwards; (b) included older adults (mean age ≥50 years); and (c) provided data on the safety and tolerability of medical cannabinoids.
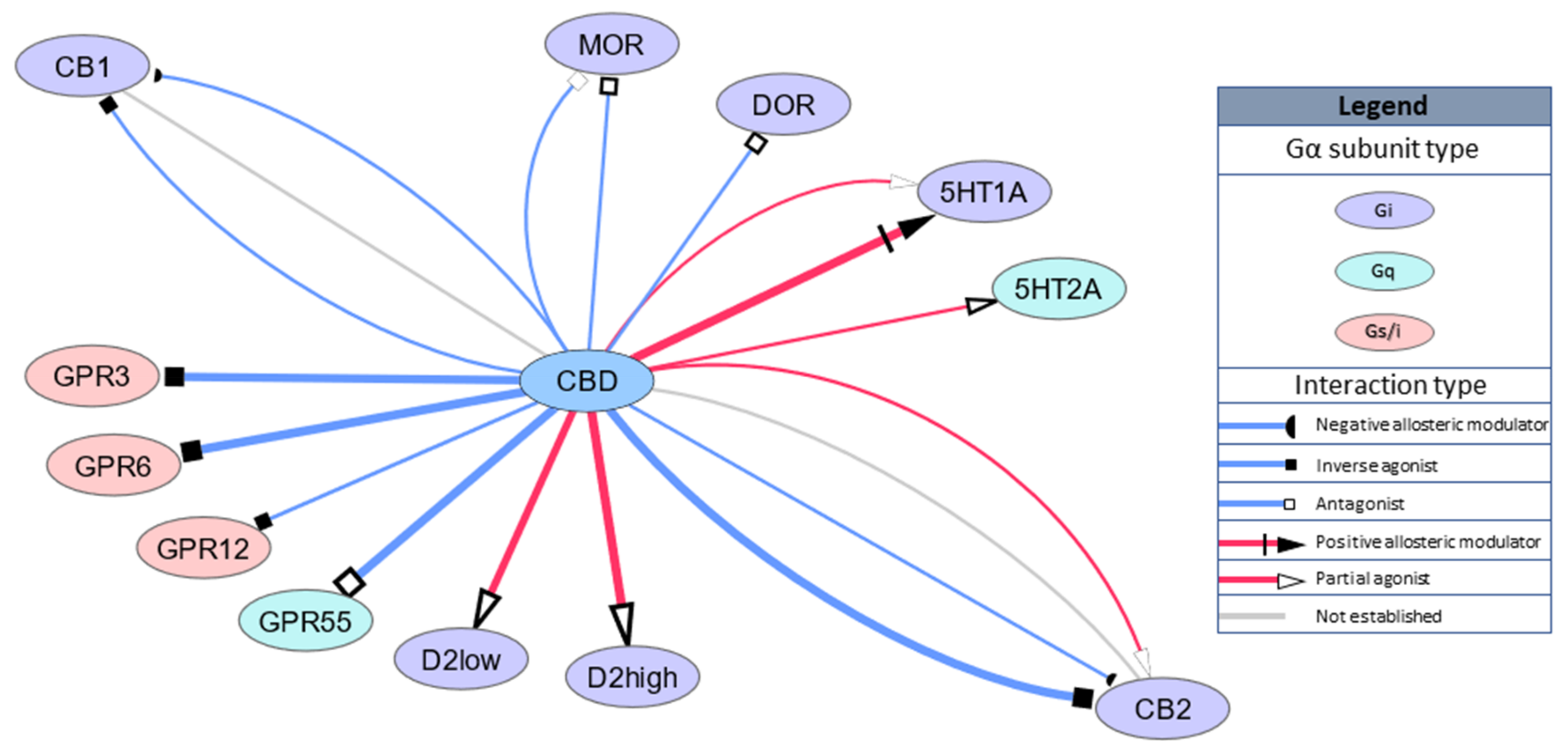
Risk of adverse events, serious adverse events and withdrawals was computed as the incidence rate (IR). Separate analyses were conducted by the cannabinoid-based medication used, for delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD) and a combination of THC and CBD (THC:CBD). RESULTS: Thirty-eight studies were identified (THC = 23; CBD = 6; THC:CBD = 9; N = 2341, mean age: 63.
08 years, men: 53. 86%). THC had a very low incidence of all-cause and treatment-related adverse events (IR: 122. 18, 95% confidence interval [CI] 38. 23-253. 56; IR: 84. 76, 95% CI 0. 13-326. 01, respectively) and negligible serious adverse events (IR = 0). Similar IRs for CBD (all cause, IR: 111.
Buy Medical Use Of Cannabis And Cannabinoids – An Update in LA

24-495. 93; treatment related, IR: 1. 76, 95% CI 4. 63-23. 05) and no serious adverse events (IR = 0). CBD was not associated with a risk of treatment-related withdrawals. THC had a low risk of all-cause and treatment-related withdrawals (IR: 25. 18, 95% CI 12. 35-42. 52; IR: 7.
26-14. 38, respectively). The THC:CBD treatment had a low risk of all-cause and treatment-related adverse events (IR: 100. 72, 95% CI 0. 25-383. 00; IR: 55. 38, 95% CI 8. 61-142. 80, respectively), but reported a risk of all-cause and treatment-related serious adverse events (IR: 21. 32, 95% CI 0.

26; IR: 3. 71, 95% CI 0. 21-11. 56, respectively), and all-cause and treatment-related withdrawals (IR: 78. 63, 95% CI 17. 43-183. 90; IR: 34. 31, 95% CI 6. 09-85. 52, respectively). Significant heterogeneity (I2 >55%) was present in most analyses. CONCLUSIONS: Although cannabinoid-based medications were generally safe and acceptable to adults aged over 50 years, these estimates are limited by the lack of a control condition and considerable heterogeneity.
Cannabidiol (Cbd) - Uses, Side Effects, And More - Webmd now available in NYC
AB - BACKGROUND AND OBJECTIVE: Although cannabinoid-based medications are increasingly used by older adults, their safety and tolerability in this age group remain unclear. The purpose of this systematic review was to examine the safety and tolerability of cannabinoid-based medications by conducting a meta-analysis of open-label observational studies of cannabinoid-based medications for all indications in individuals with a mean age of ≥50 years.
Study quality was assessed using an adapted version of the Grading of Recommendations Assessment, Development and Evaluation criteria and Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines were followed. We included studies that (a) were published from 1990 onwards; (b) included older adults (mean age ≥50 years); and (c) provided data on the safety and tolerability of medical cannabinoids.
Risk of adverse events, serious adverse events and withdrawals was computed as the incidence rate (IR). Separate analyses were conducted by the cannabinoid-based medication used, for delta-9-tetrahydrocannabinol (THC), cannabidiol (CBD) and a combination of THC and CBD (THC:CBD). RESULTS: Thirty-eight studies were identified (THC = 23; CBD = 6; THC:CBD = 9; N = 2341, mean age: 63.
Table of Contents
Latest Posts
Buy Best Heart Rate Monitors Of 2023 - Popular Science in LA - limited period only
Buy The 1 Thing That Will Make Every Workout More Effective in TEXAS - limited period only
Best Polar Heart Rate Monitor - H10 Vs H9 Vs Verity Sense now available in LA
Navigation
Latest Posts
Buy Best Heart Rate Monitors Of 2023 - Popular Science in LA - limited period only
Buy The 1 Thing That Will Make Every Workout More Effective in TEXAS - limited period only
Best Polar Heart Rate Monitor - H10 Vs H9 Vs Verity Sense now available in LA