Table of Contents
Buy An Audit On The First 400 Patients In New Zealand - Bjgp Open in TEXAS - limited time only
Persons with Parkinson's disease (PD) have progressive disabling tremor, slowness, stiffness, balance impairment, cognitive deficits, psychiatric symptoms, autonomic dysfunction, fatigue and insomnia. Tremor may interfere with necessary daily and work functions. The disorder affects approximately seven million people globally. The total economic cost in the US is around 23 billion dollars.
Cognitive impairment is a common feature and ranges from delayed recall in early stages to global dementia in up to 80% at end stage. PD with dementia has been associated with reduced quality of life, shortened survival, and increased caregiver distress. Depression, anxiety and psychosis are also common and are particularly disabling in PD, even at the earliest stages.
Anxiety affects up to 40% of patients with PD, and may predate motor symptoms by several years. The most common anxiety disorders in PD are panic attacks (often during off-periods), generalized anxiety disorder, and simple and social phobias. Psychotic symptoms vary in frequency according to the definition used. If mild forms are included, these affect up to 50% of patients.
However, hallucinations occur in all sensory domains and delusions of various types are also relatively common. The impact of psychosis is substantial in that it is associated with dementia, depression, earlier mortality, greater caregiver strain, and nursing home placement. Current therapies are inadequate. Medications have improved the prognosis of PD, but also have problematic adverse effects.
Buy Cannabidiol (Cbd) - Uses, Side Effects, And More - Webmd in NC - limited time only
Patients have heard from the internet, support groups and other sources that marijuana is helpful. Most are doing so on their own, without the supervision or even knowledge of their neurologist. In a survey conducted in the spring of 2014 in University of Colorado Movement Disorders Center (UCMDC) clinic about 5% of 207 PD patients, average age 69, reported using marijuana.
In another study Katerina Venderova and colleagues reported that 25% of PD patients had taken cannabis in the General University Hospital in Prague. PD mostly affects the elderly, and with the cognitive, psychiatric and motor problems, subjects are prone to falls. Cannabis is well documented to cause psychosis, slowness, and incoordination.
Thus cannabis is expected to be risky in persons with PD. Further, there are many components of cannabis, and the cannabis preparations being sold in Colorado vary widely in composition. There are no definitive data regarding the benefits and risks of these various preparations in PD. Studies on safety and efficacy are greatly needed to protect this fragile Colorado population.
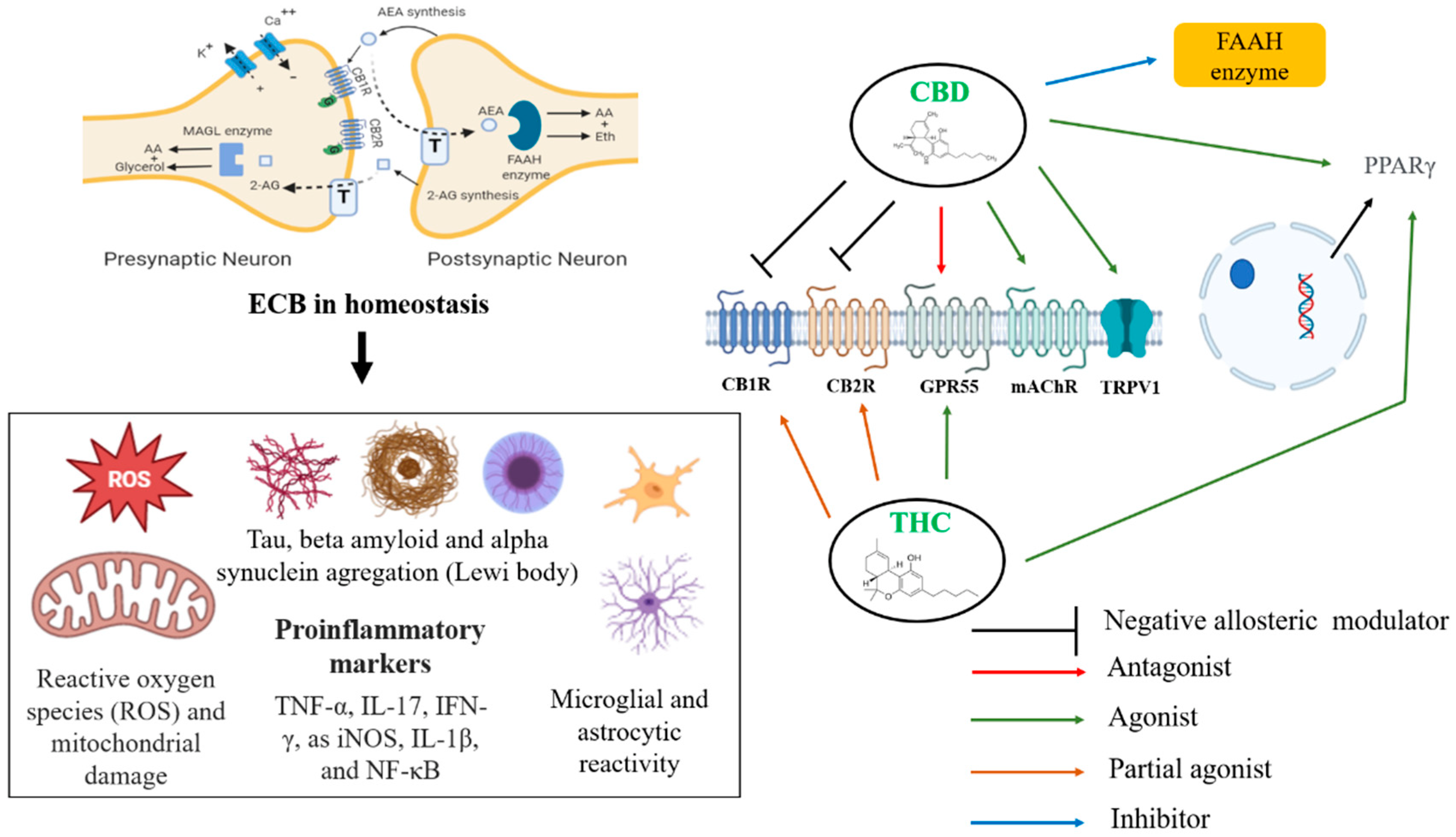
CBD acts in some experimental models as an anti-inflammatory, anticonvulsant, anti-oxidant, anti-emetic, anxiolytic and antipsychotic agent, and therefore has potential beneficial medical uses. Further, animal studies suggest that CBD is neuroprotective, perhaps due to reported anti-oxidative and anti-inflammatory actions. Human trials report that CBD decreases anxiety and causes sedation in healthy individuals, decreases psychotic symptoms in schizophrenia and PD, and improves motor and non-motor symptoms and alleviates levodopa-induced dyskinesia in PD.
Cannabidiol (Cbd) - Uses, Side Effects, And More - Webmd now available in NYC

strains with higher concentrations of THC and lower concentrations of CBD. Many clinicians who suspect cannabis may have a positive effective upon a particular patient group have no idea of the cannabinoid profile that is being used. Without knowing the composition, it is impossible to draw any conclusions simply because of the huge variety of strains utilised.
First the investigators will perform an open label study to determine a reasonable dose, and then a randomized, double-blind, placebo-controlled crossover study to evaluate the efficacy and tolerability of oral CBD on tremor and other important aspects of PD. A strength of the study is that it uses well defined form or CBD.
GWP42003-P is started at 5 mg/kg/day and is increased by 5 mg/kg at 3 day intervals to a target dose of 25 mg/kg/day. Secondary Specific Aim: To examine the effect of CBD on severity & duration of tremor and other conditions that are problematic in PD. The dose escalation tolerability study will be conducted in 10 subjects (the investigators will be recruiting up to 15 subjects to end up with 10) as an open label study lasting approximately 3 weeks followed by a 2-week safety follow up.
Subjects will have a screening visit, a baseline visit within the next three weeks, a visit when subjects are on 20 mg/kg/day, a final assessment visit when subjects have been on the maximal tolerated or the targeted dose for 10-15 days, and a safety visit 2 weeks later. The subject is to be on the maximal tolerated or targeted dose for 10-15 days.
Buy Debate Continues Over Cbd Therapy - Dvm360 in NC - limited time only
During phone calls subjects are monitored for adverse events, especially excessive daytime sleepiness, symptoms of hepatotoxicity, as well as changes in medical history and concomitant medications. Subjects are also called 3 days after stopping the study drug to check for signs of withdrawal..
Table of Contents
Latest Posts
Buy Best Heart Rate Monitors Of 2023 - Popular Science in LA - limited period only
Buy The 1 Thing That Will Make Every Workout More Effective in TEXAS - limited period only
Best Polar Heart Rate Monitor - H10 Vs H9 Vs Verity Sense now available in LA
Navigation
Latest Posts
Buy Best Heart Rate Monitors Of 2023 - Popular Science in LA - limited period only
Buy The 1 Thing That Will Make Every Workout More Effective in TEXAS - limited period only
Best Polar Heart Rate Monitor - H10 Vs H9 Vs Verity Sense now available in LA